Abstract
Background: Hematopoietic stem and progenitor cell (HSPC) transplant is an essential treatment for a variety of blood disorders and malignancies, and more recently, for gene editing therapies for diseases such as sickle cell anemia. A key step in this procedure is the mobilization of donor stem cells. The most commonly used regimen for donor mobilization is a 5-day course of Granulocyte Colony Stimulating Factor (G-CSF). However, G-CSF does not effectively mobilize a sufficient number or quality of stem cells in all donors, can have unwanted side effects, and is contraindicated in sickle cell disease. Therefore, it is desirable to identify an alternative treatment that is safe, rapid and cost effective. The cell surface integrin receptor VLA-4 (a4b1) and the chemokine receptor CXCR4 are essential for HSPC homing and retention in the bone marrow. Inhibition of these receptors rapidly mobilizes HSPCs within an hour, as opposed to the multi-day process using G-CSF. Small molecule inhibitors of VLA-4 have been developed, but have poor solubility and pharmacokinetics and none are being commercially developed. These inhibitors rapidly mobilize HSPCs into the peripheral blood of mice after a single s.c. injection, but the effect is short lived, and not long enough to collect an adequate number of HSPCs necessary for stem cell transplantation or for gene therapy. Therefore, VLA4 inhibitors with improved properties imparting extended HSPC mobilization after a single dose is desirable.
Methods: We synthesized multiple iterations of VLA-4 inhibitor molecules and tested their potency using soluble VCAM-1 binding assays. Inhibitors determined to be most potent with improved aqueous solubility were then tested in vivo in DBA mice for extended HSPC mobilization beyond 4 hours after a single injection, alone and in combination with the CXCR4 inhibitor Plerixafor or BL-8040 (n=5). HSPC mobilization was measured in wild-type and splenectomized mice via flow cytometry to quantify the proportion of LSK (Lineage- Sca+ cKit+) cells as well as via Colony Forming Unit (CFU) assays. For competitive transplantion studies, mobilized CD45.1+ BALB/c mouse blood (10 uL) was injected into lethally irradiated CD45.2+ BALB/c recipients alongside 2.5x105 CD45.2+ BALB/c bone marrow cells (n=10 / cohort). HSPC engraftment was monitored monthly via flow cytometry for ratio of 45.1+ vs. 45.2+ cells in PB.
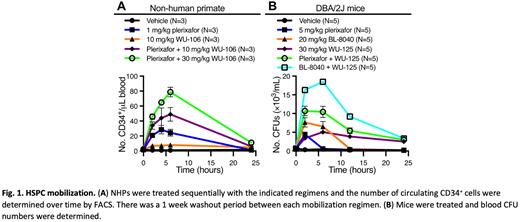
Results: We developed and optimized novel polyethylene glycol (PEG) conjugated inhibitors of VLA-4 for HSPC mobilization by strategically attaching varied PEG length chains to an optimized VLA4 inhibitor binding core. Through testing of these analogues in our mouse mobilization assay, we determined that a minimum PEG length of 24 PEG units was required for extended mobilization. This 24 PEG unit analogue, WU-106, exhibits excellent aqueous solubility and mobilizes 2-fold more murine stem cells for a longer time period than previously published best-in-class VLA-4 inhibitors or our own early VLA4 inhibitor leads. A synergistic effect is achieved when WU-106 is co-administered with a CXCR4 inhibitor (Plerixafor or Motixafortide). The efficacy of HSPC mobilization by WU-106 alone and in combination with Plerixafor in non-human primates (NHPs) was also evaluated. WU-106 alone was a weak mobilizing agent and increased the number of circulating CD34+ HSPCs 5-fold over baseline at 6 hours after injection. However, the combination of plerixafor and 30 mg/kg WU-106 induced synergistic mobilization of CD34+ HSPCs (Fig.1A). Further optimization by the addition of even longer PEG chains (WU-125) retains sub nM potency and extends mobilization beyond 24 hours after a single injection . Superior synergistic effects are observed when combined with a CXCR4 inhibitor (Motixafortide; Fig. 1B).
Conclusion: We have developed novel, potent VLA-4 inhibitors that have excellent aqueous solubility and pharmacokinetics, and which rapidly mobilize HSPCs and maintain this effect for at least 6 hours, and with some analogues out to 24 hours and greater after a single dose. When dosed in combination with the CXCR4 inhibitors Plerixafor or Motixafortide, a synergistic effect is achieved in mice and non-human primates. An ongoing competitive repopulation study in mice thus far demonstrates superior engraftment durability from HSPCs mobilized with our lead VLA4 inhibitors in combination with CXCR4 inhibitors compared to HSPCs mobilized with G-CSF.
Disclosures
DiPersio:Amphivena Therapeutics: Research Funding; NeoImmune Tech: Research Funding; Macrogenics: Research Funding; BioLineRx, Ltd.: Research Funding; CAR-T cell Product with Washington University and WUGEN: Patents & Royalties; VLA-4 Inhibitor with Washington University and Magenta Therapeutics: Patents & Royalties; hC Bioscience, Inc.: Membership on an entity's Board of Directors or advisory committees; RiverVest Venture Partners: Consultancy, Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy, Research Funding; WUGEN: Current equity holder in private company, Research Funding; Magenta Therapeutics: Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal